August 19, 2019 @ 10:00 am - 11:00 am
Event Navigation
Neurovascular Coupling : “A Balance of Power”
Prof. Tim David, Dept. of Mechanical Engineering
University of Canterbury, New Zealand
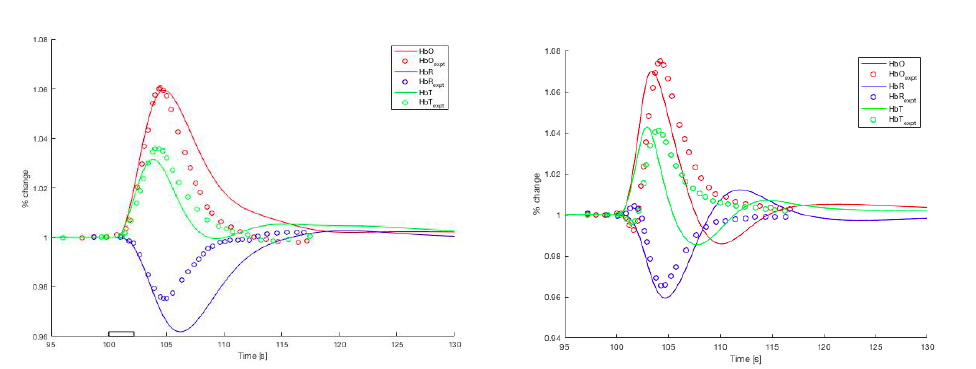
Neurovascular coupling is the ability of the cortical tissue to regulate the blood supply locally. Blood oxygen level dependent (BOLD) signals represent neuronal activity (via measurement of de-oxyhaemoglobin)and is the gold standard for functional MR protocols. We have developed a complex cellular, numerical model of neurovascular coupling utilising the neurovascular unit (NVU) consisting of neurons, astrocytes, smooth muscle cells and endothelial cells. The neuron model exhibits both excitatory and inhibitory functions whilst the other cells determine the time-dependent ion concentration profiles. This model now has the ability to simulate both time-varying cerebral blood flow and the associated fMRI BOLD responses due to continuous neuronal spiking, bursting phenomena and task orientated stimulation [1-4]. Numerical simulations are compared to experiments of the stimulation of awake rat whiskers of varying duration observed in the rat barrel cortex [5]. Comparison shows excellent agreement for both blood flow and haemoglobin profiles. The results support the theory that for somatosensory stimulation K+ is the key pathway for providing above baseline levels of oxygen and glucose to the cerebral cortex during local activity. In this case there is a balance between constrictive (via 20-HETE) and dilatory (via VOCC) pathways. However when interneurons are activated (via optogenetic methods) the K+ pathway is not activated at all and instead there is a balance between dilatory functions mediated by GABA (γ-aminobutyric acid) and constrictive actions mediated by NPY (neuropeptide Y), again a balance of power.
The model is able to conduct in-silico experiments that are either impossible or ethically restrictive in the laboratory.
Figure Caption: Left:Haemoglobin profiles compared with experimentals. Right: optogenetic interneuron profiles compared with experiments.
Acknowledgments: Funding provided by Brain Research New Zealand
References:
1) Dormanns, K.; et al ( 2015): Neurovascular coupling and the influence of luminal agonists via the endothelium, Journal of Theoretical Biology, Vol. 364 pp.49–70.
2) Mathias, E. Kenny, A.; Plank, M. J. M. and David, T. (2018): Integrated models of neurovascular coupling and BOLD signals: Responses for varying neural activations, NeuroImage, Vol. 174, pp. 69–86.
3) Mathias, E. J.; Plank, M. J. and David, T. (2017): A model of neurovascular coupling and the BOLD response: PART I, Computer Methods in Biomechanics and Biomedical Engineering, Vol. 20, pp. 508–518.
4) Kenny, A.; Plank, M. J. M. and David, T. (2018): The role of astrocytic calcium and TRPV4 channels in neurovascular coupling, Journal of Computational Neuroscience, Vol. 44, pp. 97–114.
5) Zheng, Y. et al (2010). A dynamic model of neurovascular coupling: implications for blood vessel dilation and constriction. NeuroImage, 235:1135–1147.